#ScienceWorks Ambassadors Urge Congress to pass PDUFA
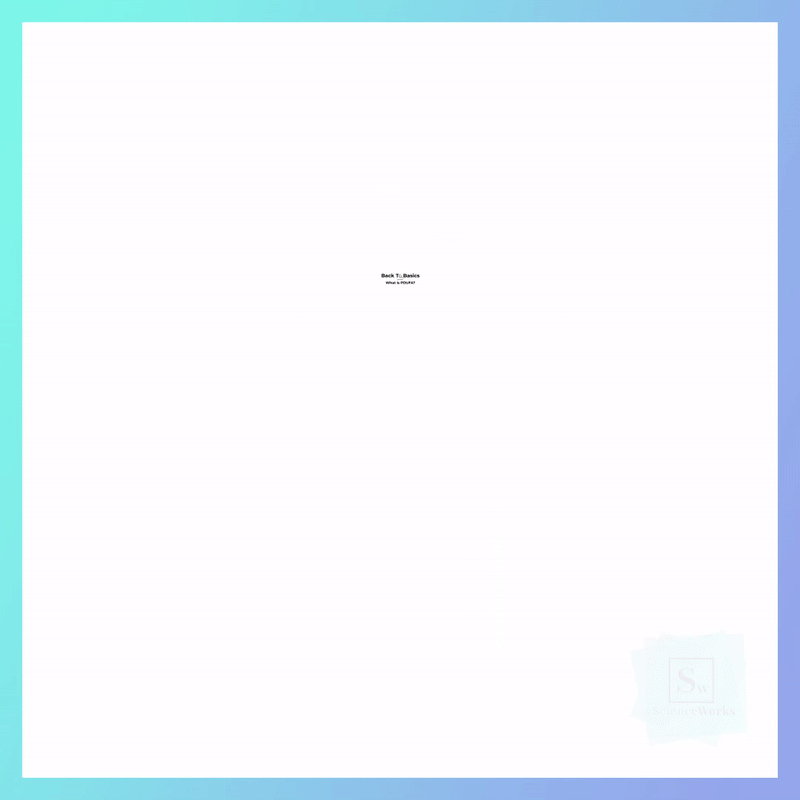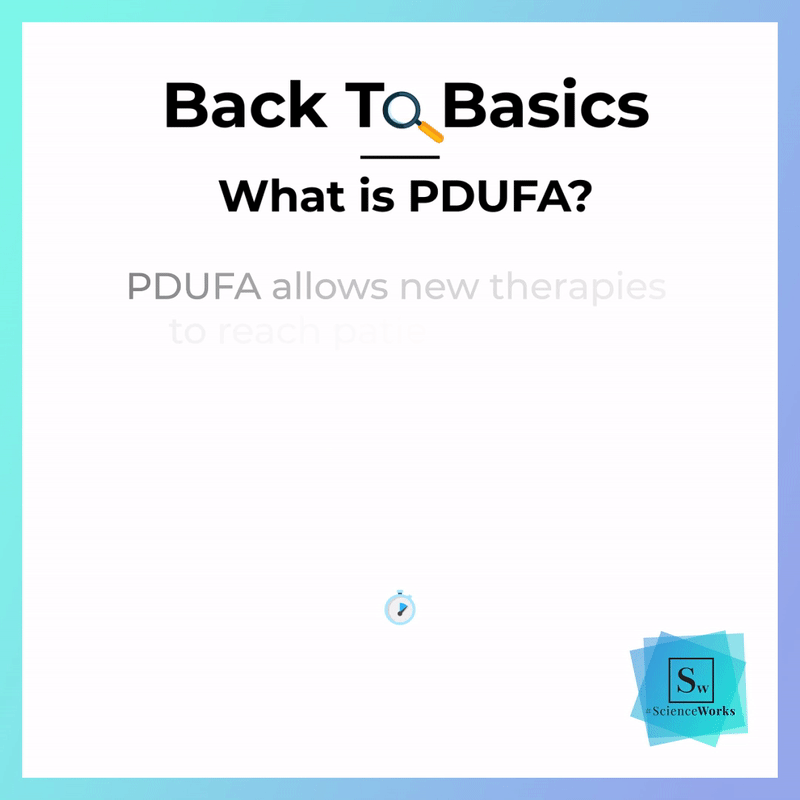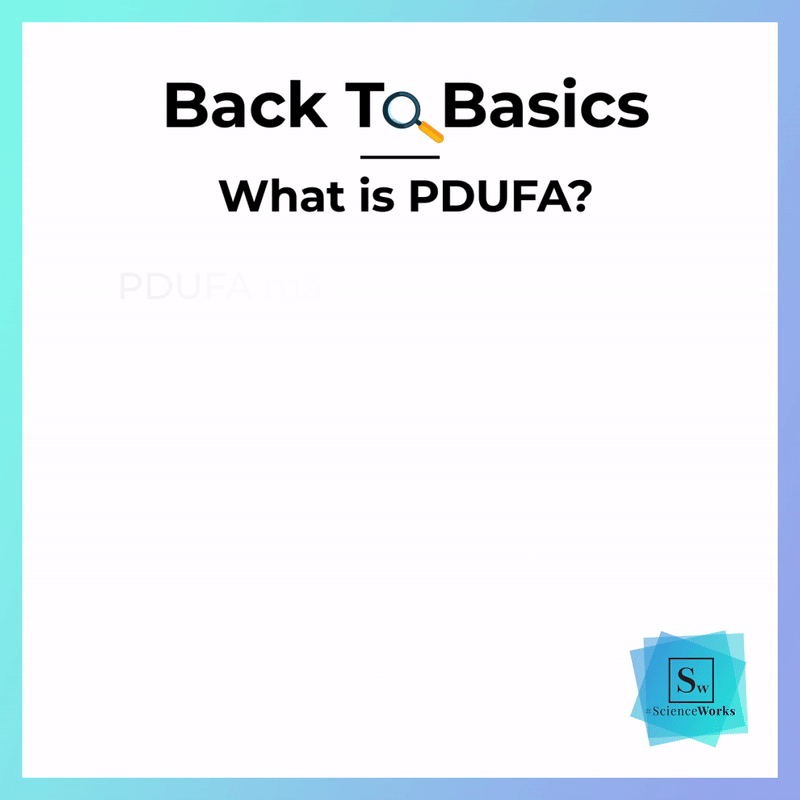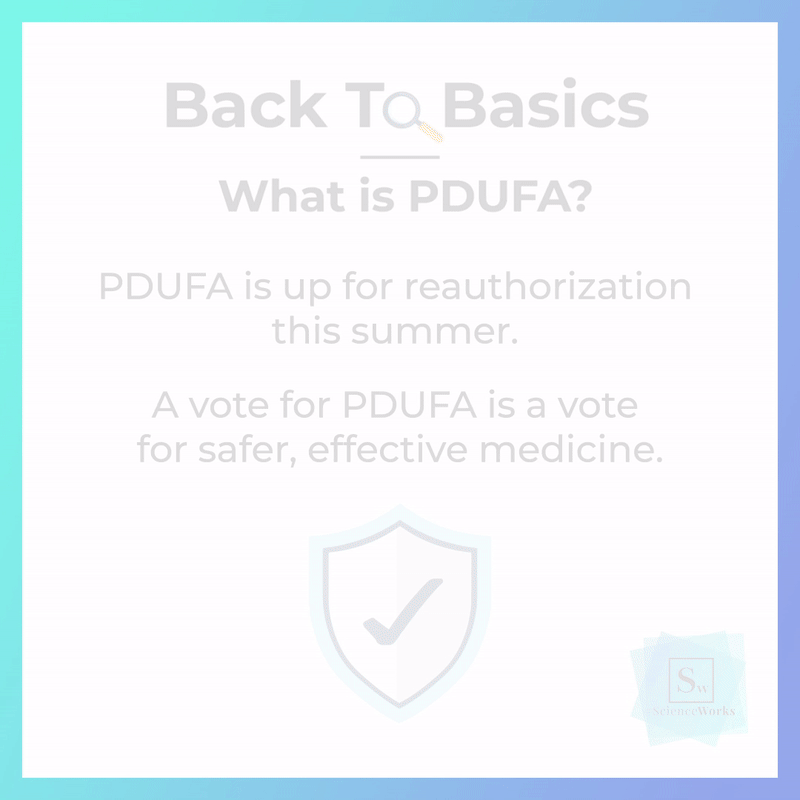
Millions of people in the U.S. rely on innovative medicines to improve their quality of life, and a federal law called the Prescription Drug User Fee Act (PDUFA) plays a critical role in ensuring they have safe and timely access to these medicines. But unless Congress reauthorizes the law this summer, PDUFA will expire and place those in need at risk.
Read More
Millions of people in the U.S. rely on innovative medicines to improve their quality of life, and a federal law called the Prescription Drug User Fee Act (PDUFA) plays a critical role in ensuring they have safe and timely access to these medicines. But unless Congress reauthorizes the law this summer, PDUFA will expire and place those in need at risk.
That is why the biopharmaceutical scientist partners of #ScienceWorks are calling on Congress to act, reauthorize PDUFA, and keep science working.
PDUFA helps ensure that breakthroughs in biopharmaceutical science receive timely and thorough consideration by the U.S. Food and Drug Administration (FDA)—so new drugs and treatments can become available to patients. PDUFA improves communications between drug sponsors and the FDA throughout the drug development process. With clearer communication, the development, review, and approval processes move forward with more clarity and predictability, increasing both safety and the speed with which innovative medicines can begin improving lives.
Thanks in large part to PDUFA, the U.S. leads the world in the introduction of new medicines. Since this bipartisan law was enacted in 1992, PDUFA has provided more timely access to over 1,700 new drugs and biologics. And over the last five years, about 75 percent of novel drugs were approved in the U.S. before any other country. The FDA’s drug review program is the gold standard for regulatory review and approval around the world.
Before PDUFA, it often took the FDA more than two years to review new medicines. Now, on average, it takes just 10 months, while still protecting public health and prioritizing quality. The reauthorization of PDUFA will allow the agency to keep pace with the number and complexity of innovative drugs and biologics entering the review pipeline.
The latest reauthorization of PDUFA in 2017 broke new ground in patient-centric drug review and ensured that the U.S. pharmaceutical innovation ecosystem could remain robust and successful. But this iteration of PDUFA ends on September 30, 2022, and must be reauthorized by Congress before it expires to ensure continued innovation, modernization, and overall improvement of the current FDA review process.
Stand with #ScienceWorks as we rally biopharmaceutical scientists across the country to ask our members of Congress to reauthorize the Prescription Drug User Fee Act this summer so we can keep science working for America.
Take Action



 Back To Top
Back To Top